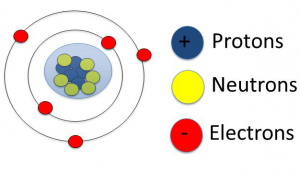
Atomic structure
Acceding to the modern electron theory, every matter in the universe is made up of very small divisible particles known as molecules. Every molecule is further composed of very minute particles called atoms.
- Every matter differs from the other in atomic structure.
- The electrical behaviour of the matter can study from its atomic structure.
The atom has two parts :
- Nucleus
- Extra-nucleus
Nucleus: The central core of the atom is known as the nucleus.
Properties of nucleus :
Nucleus contains two types of particles.
- Protons
- Neutrons
Protons: Protons are positively charged particles. each proton has a positive charge of 1.602×10-19 coulombs.
Neutrons: Neutrons have no charge at all. The mass of neutrons is equal to the mass of the proton. The mass of the nucleus is the sum of the mass of protons and neutrons contained in it. This mass of the nucleus is supposed to be the entire mass of the atom.
The nucleus has a net positive charge due to its protons.
Extra-nucleus: The outer part surrounding the nucleus is known as the extra-nucleus.
Properties of the extra-nucleus
- It consists of tiny particles known as electrons each electron has a negative charge of 1.602×10-19 coulomb.
- Thus the total mass of the atom is assumed to be opposite in polarity.
- The electrons are approximately 1/1840 times that of the protons and hence it is neglected.
- Thus the total mass of the atom is assumed to be concentrated at the nucleus.